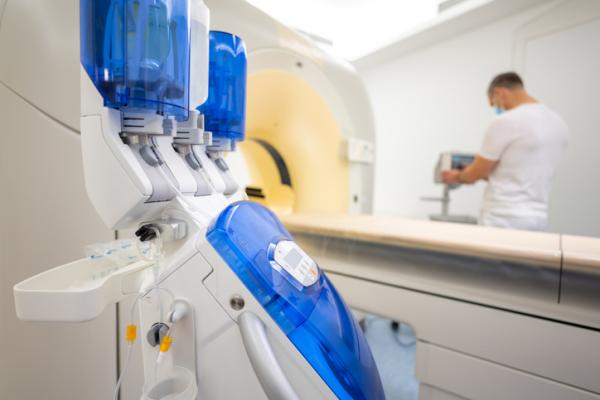
FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium
Bracco Diagnostics Inc. has announced that the U.S. Food and Drug Administration (FDA) granted import discretion of Iomeron (iomeprol injection) into the U.S. to address the ongoing iodinated contrast media shortage. The product addresses the need for the most advanced diagnostic imaging standards and will be temporarily available in the U.S. market starting at the end of August, 2022.
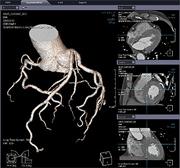
Infinitt Xelis Cardiac Software Receives FDA Clearance
S-1
articles • APPLIED RADIOLOGY
articles • APPLIED RADIOLOGY

Global Contrast Media Injectors Market to Reach $1.8 Billion by 2022

Survey Reports Radiologist Input on Key Issues, MRI Contrast Agents

EHR Interventions for Contrast Media Shortage Impact CT Utilization

Bayer Showcases MEDRAD Stellant FLEX CT Injection System

articles • APPLIED RADIOLOGY
Contrast Media Injectors







